ISO 13485 2-Stage Registration Audit
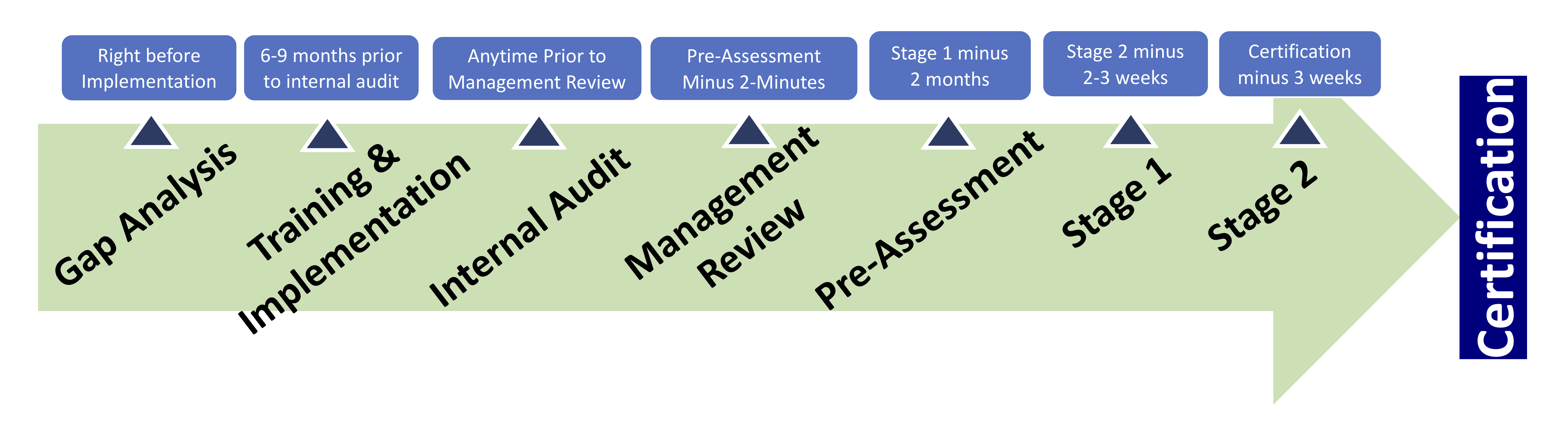
Stage 1 (Readiness Review) Audit:
The Stage 1 Readiness Review, as the name states, is the first stage in the certification audit process. Its goal is to determine if the organization is able to move on to the Stage 2 Certification Audit. During stage 1 the registrar will review the requirements of the management system, including: documented information, evaluate the client’s site specific conditions, and talk to personnel. The auditor will review the organization’s scope and gather information on the processes and operations, equipment, levels of control, and any statutory or regulatory requirements. The auditor is looking to make sure that objectives are being met and key performance indicators, or quality aspects, are defined and understood. Both internal audits and management reviews will be evaluated to make sure they are being performed adequately along with the implementation of the management system to determine if the organization is ready to move forward with the Stage 2 Certification Audit.
A Stage 1 Audit usually takes place in one or two days. This audit is almost always onsite, but when an organization has more than one location the audit may occur at their head office. After the Stage 1 audit is complete, planning for Stage 2 may begin. This includes reviewing the allocation of resources and details for the next phase of the audit. Documented information will be given to the organization to allow them to fix any nonconformances that may arise in the final audit.
Stage 2 (Certification) Audit:
The purpose of the Stage 2 Audit is to confirm a company’s quality management system is fully compliant with ISO 13485:2016. One to two months following the Stage 1 audit, the certification body (registrar) will return to audit your entire quality system. The CB will analyze each process within your organization for compliance with ISO 13485. This includes such things as customer requirements, and legal and organizational requirements. The length of the Stage 2 audit is determined by the size of the organization, number of sites, and the functions included within the system.
The Stage 2 Audit will include:
- Evaluating the documented information to ensure that the management system conforms with all standard requirements
- Report how well the quality management system complies with the organization’s quality manual and procedures
- Evaluation of internal audits, management review and management responsibility for the organization’s policies
- Report all nonconformances so that they can be assessed further.
- Create the surveillance plan for the organization and choose dates for the first surveillance visit in the following months.
If the Certification Body does not discover any serious nonconformances, the organization’s management system(s) will be certified.
Surveillance Audits
The Certification Body performs surveillance audits once per year during the issued certificate validity. Surveillance audits are performed and documented in an analogical way like the certification audit. Lead auditors shall elaborate the surveillance audit report within 14 days from the realization of surveillance audit.
Transfer of accredited certification
Transfer of accredited certification means appreciation of existing and valid management system certificate awarded by other accredited certification body for the purposes of certificate.
Registrars
Certification Bodies, otherwise known as registrars, are the auditors that come complete the stage 2 certification audit within your company. Finding ANAB accredited registrars can be done by completing the free direct quotation questionnaire. Learn more about who can grant ISO 13485 Certification here.
- Select a Certification Body/Registrar. Do this early, within the first few months into your implementation project and budget to cover the costs.
- Registrar makes use of the ISO 13485:2016 standard for Quality Management Systems Audit Requirements.
- Registrar performs Stage 1 Readiness audit including a documentation review after your procedures are finalized and approved.
- Registrar performs Stage 2 Registration audit, approximately 3 months after you have used your QMS and generated sufficient records to prove that you are in compliance.
- Address non-conformances, if any, found during the audit.
- Receive Quality Management System Certificate.
- CELEBRATE your ISO 13485 QMS Accomplishment.
- Advertise Your ISO 13485 Registration


