How to Implement ISO 13485:2016 & ISO 9001:2015 in One Management System
 The publication of ISO 13485:2016 without the adoption of Annex L has created some problems for companies working with multiple quality management systems. The primary reason the new structure was not adopted was because the planning process for this standard began before ISO 9001:2015 and other quality standards, and the old standard structure of ISO 13485:2003 was well-aligned with the regulations in most of the member countries. Because ISO 13485:2016 has a differing structure from ISO 9001:2015, the new medical standard no longer completely encompasses 9001, and organizations wishing to conform to both must address the requirements separately. Even though the new ISO 13485:2016 standard does not follow Annex L, there is still significant overlap between the standards and solutions for bridging the gap to improve operational efficiency and reduce certification cost.
The publication of ISO 13485:2016 without the adoption of Annex L has created some problems for companies working with multiple quality management systems. The primary reason the new structure was not adopted was because the planning process for this standard began before ISO 9001:2015 and other quality standards, and the old standard structure of ISO 13485:2003 was well-aligned with the regulations in most of the member countries. Because ISO 13485:2016 has a differing structure from ISO 9001:2015, the new medical standard no longer completely encompasses 9001, and organizations wishing to conform to both must address the requirements separately. Even though the new ISO 13485:2016 standard does not follow Annex L, there is still significant overlap between the standards and solutions for bridging the gap to improve operational efficiency and reduce certification cost.
Why Annex L?
Annex L is a new management system structure that helps streamline the creation of new standards and simplifies the implementation of multiple standards within one organization. Annex L structure addresses integration issues and improves the development and implementation of International Standards.
Learn More: Annex L
Why Integrate ISO 9001 and ISO 13485?
There are many situations that may benefit from certifying to multiple quality management standards, for example:
- Organizations that have an existing commercial customers who require broader quality standard (such as ISO 9001) and need to prove certification to the medical device market with a narrower standard (such as ISO 13485).
- Conversely, those organizations that have narrower standards but may wish to pursue broader quality management standards, or other management standards such as safety or environmental.
- Those organizations that have no existing standards, but think they might need multiple standards in the future and who wish to avoid having to recreate/modify their existing systems later.
In all cases, the goal of reusing as much of the system (documentation, training, internal auditing approaches, etc.) as possible without modification greatly saves time and resources. For those specifically integrating ISO 9001 and ISO 13485, even with the structural differences that now exist, there are steps that can be taken to minimize the amount/need for modification.
Integration is Easier Than You Think
ISO 13485:2016 retains the clause structure of ISO 13485:2003 and ISO 9001:2008, but because ISO 13485:2016 hasn’t changed much from its predecessor, organizations can benefit from the familiarity of the old standard as they work towards alignment with ISO 9001:2015. A cursory look at the two standards might lead an organization to believe they need two quality manuals to address the requirements of each; however, this effort isn’t necessary. While these standards differ in structure and terminology, there is enough overlap for them to be implemented simultaneously and without conflict.
One primary difference between the standards deals with how organizations consider risk. Aligning ISO 13485:2016 with Annex L structure will require the consideration of a process-based, holistic approach to addressing the needs of the entire management system. Standards Stores has been working to create products to help your organization visualize what ISO 13485:2016 would look like if it adopted Annex L with the current requirements. Our products simplify conformity for those trying to navigate the differences between ISO 13485:2016 and ISO 9001:2015 and save time and money for those eventually migrating to future versions of ISO 13485, which will adopt Annex L.
Annex B of ISO 13485:2016 offers a clause analysis of the differences between the two standards. Our ISO 13485 and ISO 9001 comparison charts may also be useful for those wishing to gain a better understanding of where the requirements converge and diverge.
Learn More: Comparison Chart of ISO 13485 & ISO 9001
Solutions That Bridge the Gap Between ISO 13485:2016 and ISO 9001:2015
There is a way to rearrange these requirements and make the two standards work together in a single quality management system and manual, and we’ve done it for you. Check out our simplified solutions below:
ISO 9001:2015 / ISO 13485:2016 Combined QMS Package – $597
This complete quality management system package offers guidance for the documentation for the manual, procedures, and forms to assist you in customizing everything to your individual business. This provides you with a complete QMD-003 Manual that meets the requirements of both ISO 9001:2015 and ISO 13485:2016.


ISO 9001:2015 to ISO 13485:2016 QMS Transition Instructions – $299
These instructions allow you to upgrade your ISO 9001:2015 Quality Management System (QMS) to include the ISO 13485:2016 requirements for the medical devices industry while retaining the High Level Structure (HLS) of Annex L. The standards are compatible with each other and have common requirements, but their structures are different.

ISO 9001:2015 to 13485:2016 Internal Audit Checklist – $125
Standards Stores offers a variety of auditing checklists to ensure your certification audit runs smoothly. Our ISO 13485:2016 Audit Checklist includes auditing planning and reporting forms, an internal audit procedure and an internal auditing guide. We also offer an ISO 13485:2016 Internal Audit Checklist with FDA Requirements, and an auditing checklist designed specifically for ISO 13485:2016 and ISO 9001:2015 combined management systems.

ISO 9001:2015 / ISO 13485:2016 Combined Gap Analysis Checklist – $69
This gap analysis checklist will help your organization evaluate your Quality Management System (QMS) as you transition from ISO 9001:2015 to include ISO 13485:2016 and outline areas that need improvement to meet the requirements of both standards.
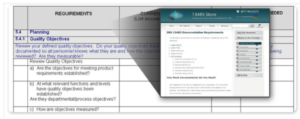
Caption for image of standard Annex B: Annex B of ISO 13485:2016 offers a detailed clause analysis of the similarities and differences between ISO 13485:2016 and ISO 9001:2015. This will help your organization identify exactly where the discrepancies lie and pinpoint ways to address them. The above graphic is copyrighted material by the International Organization for Standardization (ISO).


