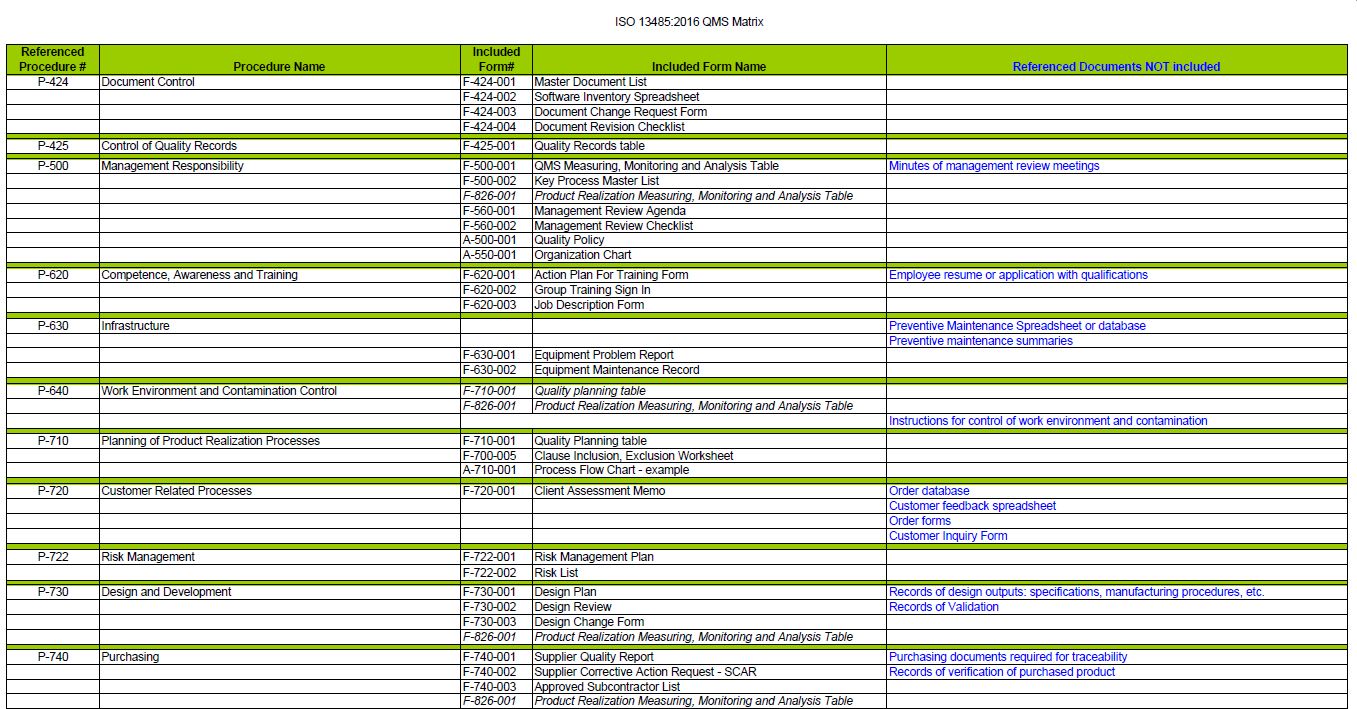
A proper QMS should have forms referenced in the procedures. This package of ISO 13485 Forms is a complete set of forms & tables to complete your ISO 13485 Quality Management System.
They are written in Microsoft Word and Excel format and are ready to customize for your organization.
The forms record the data to measure the effectiveness of a procedure. So every requirement will need to have a form.
These forms have been designed to be integrated with our Quality Manual and Procedures. Save by purchasing the Quality Manual, Procedures and Forms package.
Complete set of ISO 13485:2016 Forms
| 13485:2016 Forms |
|---|
|
Compare Products
Which version is right for me?
- Choose 13485:2016 to create a new 13485 Quality Management System
- Choose 13485:2003 to 13485:2016 QMS instructions to modify your existing 13485 QMS to meet the 13485:2016 requirements
- Choose 13485:2016 FDA QMS Upgrade to develop an integrated management system that is 13485 and FDA.QSR (21 CFR 820) compliant as of April 2016 (this is the reference date of the CFR)
- Choose ISO 9001:2015 / 13485:2016 QMS to align ISO 13485:2016 (8 section format) with ISO 9001:2015 (10-Section Annex L Format).
-
- This QMS is for organizations (like Contract Manufacturers) who want ONE QMS for both ISO 9001:2015 and ISO 13485:2016.
- Quality Management System Templates covering both the ISO 9001:2015 (Annex L 10-section format) and ISO 13485:2016 (8-Section format) in ONE combined, Annex L manual.
If you plan to reconfigure your existing quality manual completely by yourself, you can use either of the Upgrade Instructions to create everything on your own. They will tell you where to make the changes but will NOT provide any procedure or form templates for the new requirements.
| 13485:2016 Forms Package |
13485:2016 Quality Manual & Procedures Package |
|
|---|---|---|
| ISO 13485:2016 Quality Manual (not sold separately) |
||
| ISO 13485:2016 Procedures & Attachments | ||
| ISO 13485:2016 Forms | ||
| ISO 13485:2016 Flow Charts | ||
| $109.00 Order Now! |
$497.00 Order Now! |