Save time and money with our fully customizable Quality Manual and Procedures Package. Our processes are well-organized and carefully designed to work together to lead your organization to continuous improvement.
- Documents in Microsoft Word or Excel for easy customization.
- Together, the docs include the content required to address each requirement of the ISO 13485:2016 Standard.
- Very clear instructions in obvious Blue Text to show you where to customize your Manual and Procedures. Treat the text in blue as “revisions” or information that is specific to your company.
- Docs are all numbered for an effective control of documented information and are integrated to work together in a seamless system.
- Related documents are referenced for effective record keeping.
- Intuitive architecture for easy Document Control. We’ve numbered QMS documents to correspond with the sections of the 13485 Standard. Registrars LOVE this feature!
- This is a downloadable product.
As you implement your ISO 13485 system you will also need checklists and training. You can save time and money by purchasing our Certification Packages!
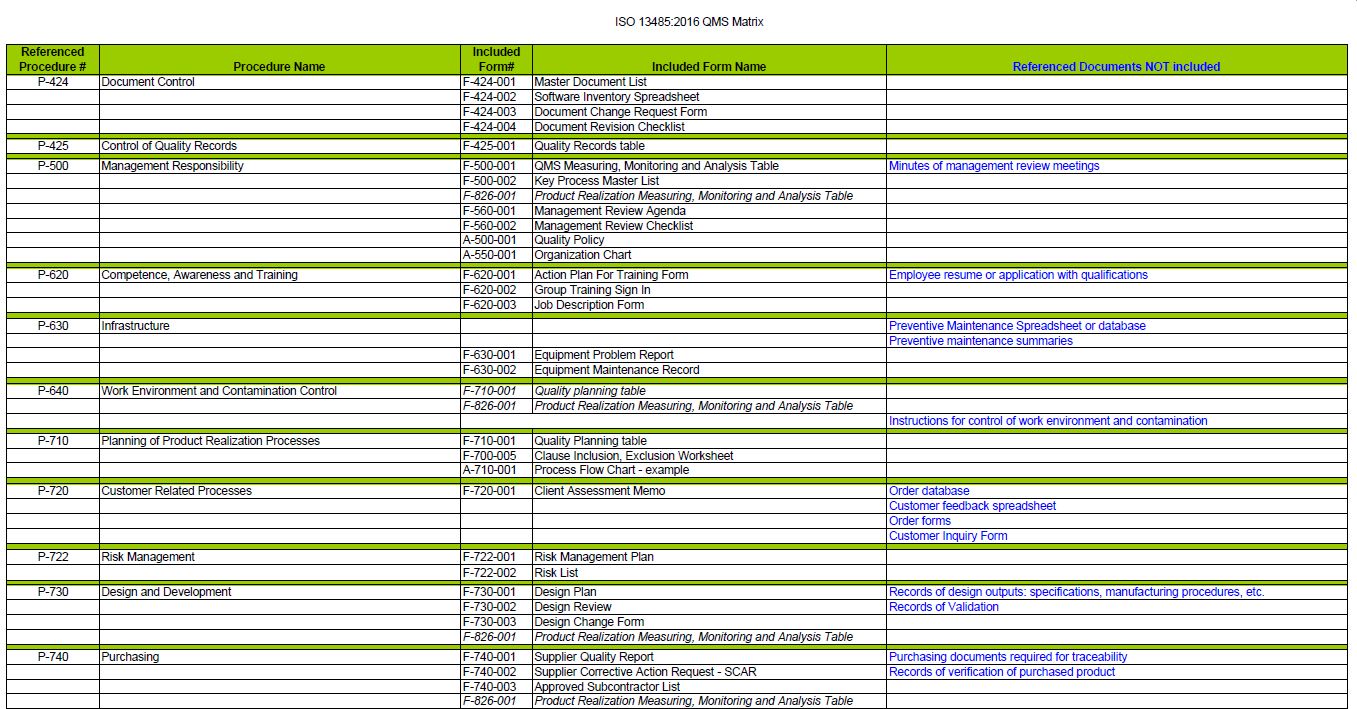
The documentation package for the management system will contain:
- (1) condensed Manual to introduce the documented information required for ISO 13485:2016.
- (26) procedures outlining how you will control each requirement of the standard.
- (42) forms to record the data required by the procedure.
- (3) attachments.
- (3) blank, formatted templates to create your own additional documents.
- (23) Flow Diagrams.
- Technical Support.
*Includes 1 Free ISO 13485:2016 Online Training (30-days to complete training).
Click below for a sample of the Quality Manual, Procedures, and Forms:
Compare Products
Which version is right for me?
- Choose 13485:2016 to create a new 13485 Quality Management System
- Choose 13485:2003 to 13485:2016 QMS instructions to modify your existing 13485 QMS to meet the 13485:2016 requirements
- Choose 13485:2016 FDA QMS Upgrade to develop an integrated management system that is 13485 and FDA.QSR (21 CFR 820) compliant as of April 2016 (this is the reference date of the CFR)
- Choose ISO 9001:2015 / 13485:2016 QMS to align ISO 13485:2016 (8 section format) with ISO 9001:2015 (10-Section Annex L Format).
-
- This QMS is for organizations (like Contract Manufacturers) who want ONE QMS for both ISO 9001:2015 and ISO 13485:2016.
- Quality Management System Templates covering both the ISO 9001:2015 (Annex L 10-section format) and ISO 13485:2016 (8-Section format) in ONE combined, Annex L manual.
If you plan to reconfigure your existing quality manual completely by yourself, you can use either of the Upgrade Instructions to create everything on your own. They will tell you where to make the changes but will NOT provide any procedure or form templates for the new requirements.
| Price Each | 13485:2003 to 13485:2016 QMS Instructions | 13485:2003 to 13485:2016 QMS Package | 13485:2016 QMS | 13485:2016 QMS with FDA | 13485:2016 / 9001:2015 Combined QMS | |
|---|---|---|---|---|---|---|
| Your Price | ||||||
| ISO 13485:2016 Quality Manual (not sold separately) | $100.00 | |||||
| ISO 13485:2003 to 2016 Upgrade Instructions | $299.00 | |||||
| ISO 13485:2016 to FDA Upgrade Instructions | $299.00 | |||||
| ISO 13485:2016 / 9001:2015 Combined QMS | $597.00 | |||||
| ISO 13485:2016 Procedures | $400.00 | 26 | 26 | 27 | 24 | |
| ISO 13485:2016 Forms and Attachments | $109.00 | 45 | 45 | 47 | 60 | |
| ISO 13485:2016 Flow Charts | $109.00 | 23 | 23 | |||
| * ISO 13485:2016 Online Training | $49.00 | |||||
| Support | FREE | |||||
| Your Price |
*Includes 1 Free ISO 13485:2016 Online Training (30 days to complete training).