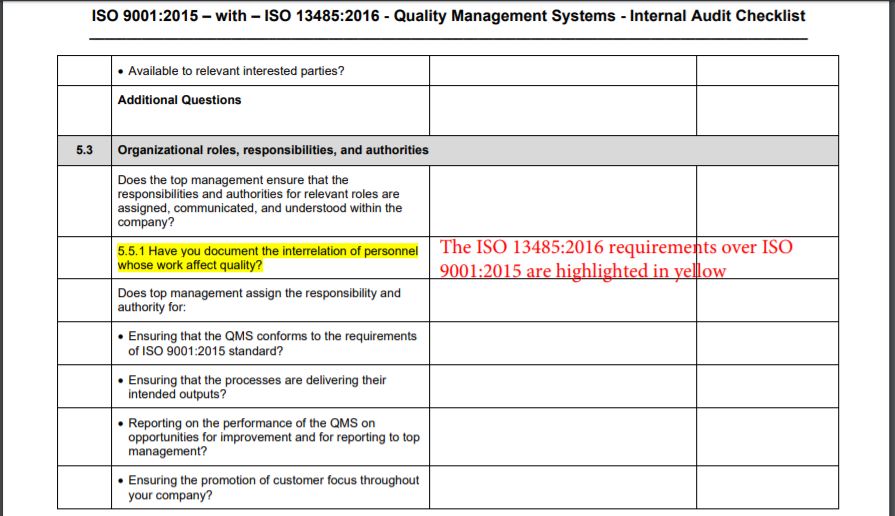
While the editions of the standards do not line up when comparing the contents and requirements, the ISO 13485:2016 requirements over ISO 9001:2015 are highlighted in yellow and the relevant ISO 13485:2016 clause number appears with the audit question. The auditors are expected to keep in mind that while ISO 13485:2016 requires specific procedures for some QMS processes, the ISO 9001 standard does not require such mandatory procedures; however, the auditors will expect documented information to be available because in the clauses of the standard, the phrase such as ‘documented procedures’ is used to specify that a process, a method, a system, a work instruction, or an arrangement be documented.
The auditors must use a great deal of discretion and therefore must be careful and thoughtful prior to establishing a deficiency against a requirement. Evidence for visible top management leadership, commitment and quality management action must be looked for.
The bold numbers and tittles used in the first two columns of the checklist indicate the “Requirements” and may be referred to on nonconformity reports prepared by the auditor.
During the assessment of each requirement, auditors record the status of the evaluation by indicating in the right-hand column a Yes – for Acceptable Condition or No – for Deficient Condition
You don’t want to cut corners with audits – buy a complete solution! Need to train your internal auditors? Buy our Internal Audit Checklist together with Internal Auditor Training Materials and save.
- ISO 13485:2016/ ISO 9001:2015 Internal Audit Checklist
- 71 Page Checklist in Microsoft Word
- An Internal Audit Procedure
- Internal Audit Forms
- F-824-001 Audit Plan
- F-824-002 Internal Audit Report
- F-824-003 Procedure by Work Area
- F-824-004 Audit Checklist
- 24 Slide PPT (15 min) outlines the basics of auditing as a foundation
- Types of Audits
- Why Audit?
- Who Can Audit?
- How to perform an Internal Audit
Compare Products
Which version is right for me?
- ISO 13485:2016 Internal Audit Checklist is for those auditing an ISO 13485:2016 Management System, and addresses each requirement of ISO 13485:2016. See the informational box above for samples, contents, and more information.
- The ISO 13485/FDA Audit checklist adds FDA QSR (21CFR-820) April 2016 requirements and highlights them for ease of identification.
- Choose ISO 9001:2015 to 13485:2016 checklist if you want the 13485 requirements highlighted – differentiating them from ISO 9001:2015
|
ISO 13485:2016 Internal |
ISO 13485:2016 with FDA Internal Audit Checklist |
ISO 13485:2016 / 9001:2015 Combined | |
|---|---|---|---|
| Audit Checklist in MS Word | |||
| Internal Audit Procedure | |||
| 3 Audit Planning Forms | |||
| Audit Report Form | |||
| Guide to Internal Audits 24-slide PowerPoint | |||
| Highlights for ISO 13485:2016 Plus FDA Requirements | |||
| Combines 13485:2016 with 9001:2015 | |||
| ISO 13485:2016 $125.00 Order Now! |
ISO 13485:2016 with FDA $125.00 Order Now! |
ISO 13485:2016 / 9001:2015 $125.00 Order Now! |