The gap analysis checklist is a very useful tool in evaluating your Quality Management System (QMS) as you transition from ISO 9001:2015 to include ISO 13485:2016. Requirements are expressed as questions for the user (auditor / assessor) to use in determining your QMS capabilities.
While the versions of the standards do not line up when comparing the requirements:
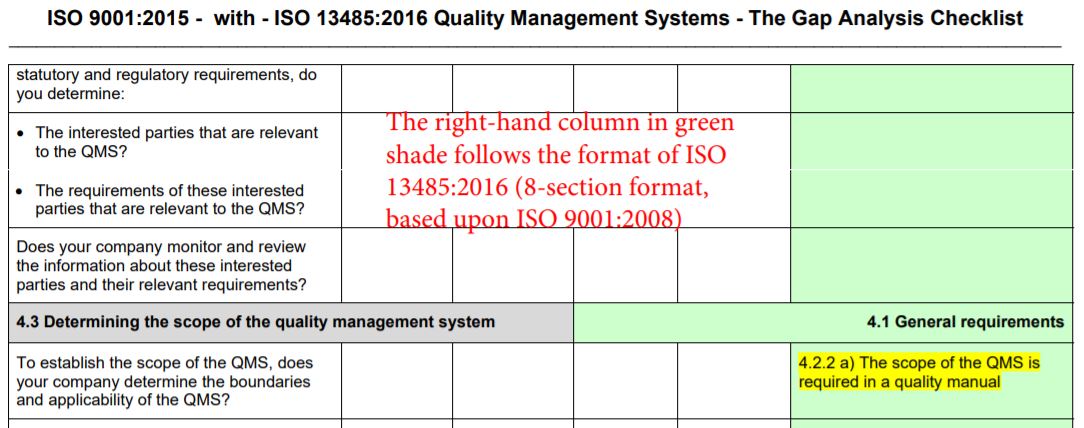
- The right-hand column in green shade is intended to provide reference / comparison / similarities to the ISO 13485:2016 requirements, and to identify and locate where in the former requirements are relevant.
- In the green shaded right-hand column, the ISO 13485:2016 requirements over ISO 9001:2015 are highlighted in yellow.
- The intent of the main clauses of the ISO 9001:2015 standard is shown in bold blue font.
For a complete set of ISO 13485:2016 documentation, visit the 13485 Store, We have designed and documented a Quality Management System for you to use as the foundation of your documentation system. This system addresses all of the requirements of the standard, from setting quality objectives and measurement criteria for your processes to internal audits and continual improvement. All the procedures interrelate to provide you with an efficient, effective quality management system.
- You know where you are (Current Quality Management System).
- You want to be ISO 13485 Compliant.
- The difference between these is your gap. Anytime they differ you need to change your processes to bridge the gap.
All for only $69.00! That’s like getting an electronic GPS for the price of a paper map!
- Includes Technical Support
- 76 page gap checklist in ISO 13485:2016 (8 section format) with ISO 9001:2015 (10-Section Annex SL Format)
Compare Products
Which version is right for me?
- Choose 13485:2016 to create a new 13485 Gap Checklist
- Choose 13485:2003 to 13485:2016 Gap Checklist to modify your existing 13485 standard to meet the 13485:2016 requirements
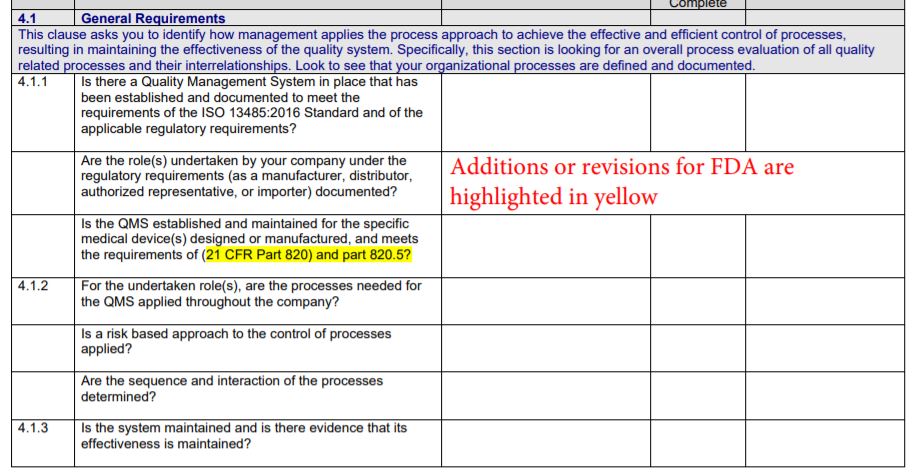
- Choose 13485:2016 FDA Gap Checklist to develop an integrated management system that is 13485 and FDA.QSR (21 CFR 820) compliant as of April 2016 (this is the reference date of the CFR)
- Choose ISO 9001:2015 / 13485:2016 Gap Checklist to align ISO 13485:2016 (8 section format) with ISO 9001:2015 (10-Section Annex L Format)
If you plan to reconfigure your existing quality manual completely by yourself, you can use either of the Upgrade Instructions to create everything on your own. They will tell you where to make the changes but will NOT provide any procedure or form templates for the new requirements.
| ISO 13485:2016 | ISO 13485:2003 to 2016 Transition |
ISO 13485:2016 FDA |
ISO 13485:2016 / 9001:2015 Combined |
|
|---|---|---|---|---|
| Current Standard: | None | 13485:2003 | FDA 2016 | 9001:2015 |
| Counts against purchase price of larger package |
||||
$49.00 Order Now! |
$69.00 Order Now! |
$69.00 Order Now! |
$69.00 Order Now! |