This list has been prepared for you by the 13485 Store. You will need to have a copy of the ISO 13485:2016 Standard to use along with this checklist. You will see questions on the checklist that refer to the standard where each requirement is expressed as a question. This checklist is based on the information provided in the 2016-03-01 release of the ISO 13485:2016 international standard.
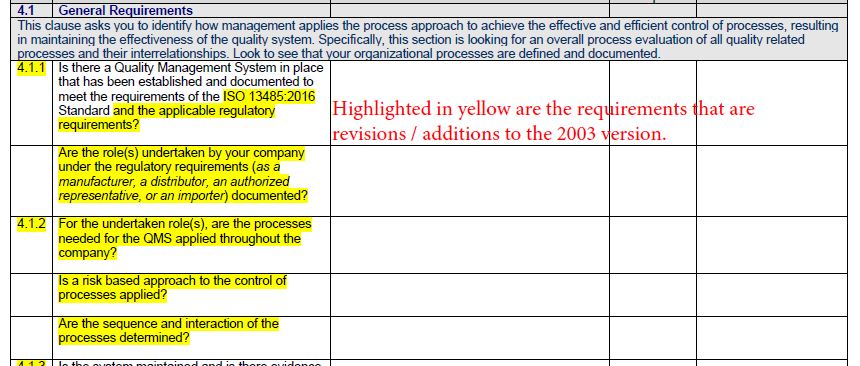
You have the ISO 13485:2003 quality management system in place and to help you with the implementation of ISO 13485:2016, we have highlighted in yellow the requirements that are revisions / additions to the 2003 version.
Keep in mind that the standard requires six (6) mandatory procedures. In the checklist, we have highlighted in Bold letters where a documented procedure is required, such as with clauses 4.2.4, 4.2.5, 8.2.4, 8.3, 8.5.2, and 8.5.3. For other clauses of the standard, the phrase such as ‘documented procedures’ is used to specify that a process, a method, a system, a work instruction, or an arrangement be documented. For your purposes, you may apply the most appropriate word.
- Includes Technical Support
- 61 Page Gap Analysis Checklist
Compare Products
Which version is right for me?
- Choose 13485:2016 to create a new 13485 Gap Checklist
- Choose 13485:2003 to 13485:2016 Gap Checklist to modify your existing 13485 standard to meet the 13485:2016 requirements
- Choose 13485:2016 FDA Gap Checklist to develop an integrated management system that is 13485 and FDA.QSR (21 CFR 820) compliant as of April 2016 (this is the reference date of the CFR)
- Choose ISO 9001:2015 / 13485:2016 Gap Checklist to align ISO 13485:2016 (8 section format) with ISO 9001:2015 (10-Section Annex L Format)
If you plan to reconfigure your existing quality manual completely by yourself, you can use either of the Upgrade Instructions to create everything on your own. They will tell you where to make the changes but will NOT provide any procedure or form templates for the new requirements.
| ISO 13485:2016 | ISO 13485:2003 to 2016 Transition |
ISO 13485:2016 FDA |
ISO 13485:2016 / 9001:2015 Combined |
|
|---|---|---|---|---|
| Current Standard: | None | 13485:2003 | FDA 2016 | 9001:2015 |
| Counts against purchase price of larger package |
||||
$49.00 Order Now! |
$69.00 Order Now! |
$69.00 Order Now! |
$69.00 Order Now! |